Classification of Hydrocarbons
Classification of Hydrocarbons: Overview
This topic covers concepts, such as, Hydrocarbons, Saturated Hydrocarbon, Unsaturated Hydrocarbon & Degree of Carbon Atoms etc.
Important Questions on Classification of Hydrocarbons
Give some examples of saturated hydrocarbons.
Unsaturated hydrocarbons are compounds that have a single bond between two adjacent atoms of carbon.
In which of the following compounds double bonds alternate between carbon atoms of the benzene ring?
Which of the following elements are present in compounds known as hydrocarbons?
How many bonds are present in ethane molecule?
Give an appropriate term for the following: Compounds containing carbon and hydrogen only. (Hydrocarbons/Halogens)
What is the test for unsaturation?
Name the most important natural source of hydrocarbon. (Petroleum / Water / Soil)
The number of and atoms in -ethyl-- methyl heptane, respectively, is:
Which one of the following is not an organic compound?
Number of carbon and hydrogen respectively in the following structure are -
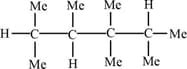
The number of tertiary atoms in tetramethyl pentane is
The number of secondary hydrogens in 2, 2-dimethyl butane is
2, 3-dimethyl hexane contains ..... tertiary ..... secondary and ......primary carbon atmos, respectively
The number of primary, secondary and tertiary hydrogens in the following compound are, respectively
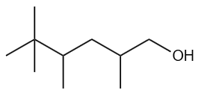
Of the following unsaturated hydrocarbons are
Match the compounds in column I with their structure(s)/ characteristic(s)/test(s)/reaction(s)/stereochemistry,etc., given in column II. Matching can be one or more than one.
| Column I | Column II | ||
|---|---|---|---|
| S.No. | Compound | Nature of H atoms | |
| a. | 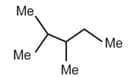 |
p. | 15(1o H), 4(2oH), 1(3oH) |
| b. | 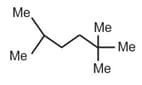 |
q. | 17(1o H), 2(2o H), 2(3o H) |
| c. | 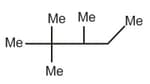 |
r. | 12(1o H), 2(2oH), 2o(3o H) |
| d. | 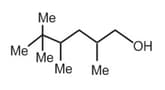 |
s | 15(1o H), 2(2o H), 1o(3o H) |
Match the compounds in column I with their structure(s)/ characteristic(s)/test(s)/reaction(s)/stereochemistry,etc., given in column II. Matching can be one or more than one.
| Column I | Column II | ||
|---|---|---|---|
| S. No. | Compound | Structure | |
| a. | C8H18 with only 1o H atoms | p. | 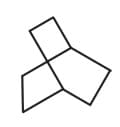 |
| b. | C6H12 with only 2o H atoms | q. | 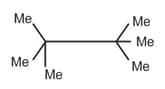 |
| c. | C6H12 with only 1o and 2o H atoms | r. | 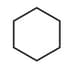 |
| d. | C8H14 with 12 secondary and 2 tertiary H atoms | s. | 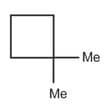 |
